Explain in Terms of Electronegativity Differences Why a C-o
Group 1 elements have an average electronegativity of 084 not including hydrogen. Therefore each P-O bond is somewhat polar.

Electronegativity And Bonding Video Khan Academy
24Base your answer to the following question on the information below and on your knowledge of chemistry.

. Explain in terms of electronegativity difference why a C-H bond is less polar than a N-H bond. Explain in terms of electronegativity differences why a CO bond is more polar than a CH bond. The boiling point of 2-propanol is 823C at standard pressure.
Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Up to 24 cash back Explain in terms of electronegativity differences why a CO bond is more polar than a CH bond. 2 on a question Explain in terms of electronegativity difference why the bond in a molecule of hf is more polar than the bond in a molecule of hi.
Electronegativity difference in a carbon-oxygen bond is greater than the electronegativity difference in a fluorine-fluorine bond The EN difference for C and O is 09 and the EN difference for F and F is 0. Therefore C-atom in CF 3 I is more electronegative than in CH 3 I. Explain in terms of electronegativity differences why a CO bond is more polar than a CH bond.
The electronegativities of oxygen hydrogen and nitrogen are. Base your answers to questions 18 and 19 on the balanced equation 2Nas Cl2g --- 2NaCls. The CO bond is more polar because the electronegativity difference for a CO bond is 08 and the electronegativity difference for a CH bond is 04.
There is a greater END in a CO bond than in a CH bond. Explain in terms of electronegativity differences why a C O bond is more polar than a C H bond. Determine the number of electrons in the bonds between the nitrogen 1 point.
The CH bond has a smaller difference. 1 point Your answer 3. The electronegativity of an atom depends upon the nature of the substituent attached to that atom.
Values for electronegativity run from 0 to 4. Write the chemical formula for one compound in the equation that contains both ionic and covalent bonds. The boiling point of 2-propanol is 823C at standard pressure.
Electronegativity difference between nitrogen and hydrogen 304 - 21 094. It is a symmetrical molecule. I think I remember that O is about 35 and P is about 21.
Thus the ion will be. In this way a polar bond is produced the polarity of which will be as the electronegativity difference between the bonded atoms is greater. 7Base your answer to the following question on the information below and on your knowledge of chemistry.
The boiling point of 2-propanol is 823C at standard pressure. There is a greater electronegativity difference in a CO bond than in a CH bond. Explain in terms of electronegativity why a CO bond in CO2 is more polar than the F-F bond in F2.
The difference in electronegativity of an atom caused by substituents results in different chemical behaviour of. Your answer 4. Electronegativity difference between oxygen and hydrogen 344 - 21 134.
Up to 24 cash back alcohol solution contains 2-propanol and water. The electronegativity values of C and O are different and produce a polar bond while the two fluorine atoms have the same electronegativity values and produce a. It can also be used to predict if the resulting molecule will be polar or nonpolar.
The boiling point of 2-propanol is 823C at standard pressure. 71 Explain in terms of electronegativity differences why a C O bond is more polar than a C H bond. Experiments have determined that the.
Explain in terms off electronegativity differences why a C-O bond is more polar than a C-H bond. There is a greater electronegativity difference in a CO bond than a CH bond. Look up the electronegativity of P and O.
Explain in terms of electronegativity why a C-O bond in CO2 is more polar than the F-F bond in F2. Explain in terms of electronegativity differences why a CO bond is more polar than a CH bond. 1 Answer There is a greater electronegativity difference in a CO bond than in a CH bond.
A metal M was obtained from a compound in a rock sample. Alcohol solution contains 2-propanol and water. Group 17 elements have an average electronegativity of 299.
For example the carbon atom in CF 3 I acquires a greater positive charge than CH 3 I. When two atoms have different electronegativities the one with the highest electronegativity will attract the electrons towards each other generating a separation between the positive and negative charges. 3 The CO bond has a greater electronegativity difference than a CH bond.
More the electronegativity difference more polar is the bond. The CO is 8 and the CH is 4. Explain in terms of distribution of charge why a molecule of the substance represented in diagram 3 is nonpolar.
The electronegativity is different in a C-O bond because 08 and the electronegativity is different in a C-H bond is 04 which is less polar Which quantities.
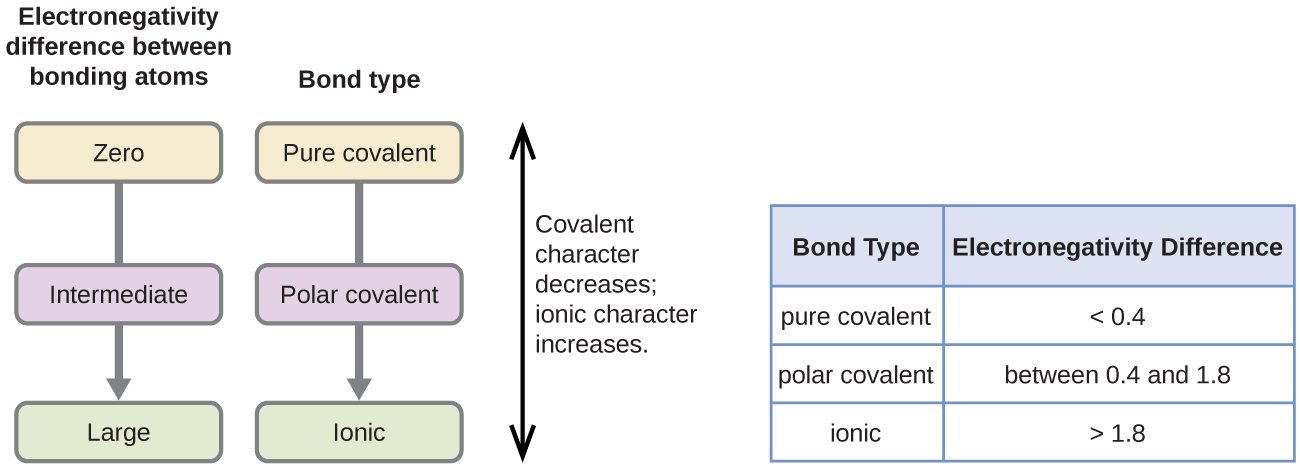
7 2 Covalent Bonding Chemistry
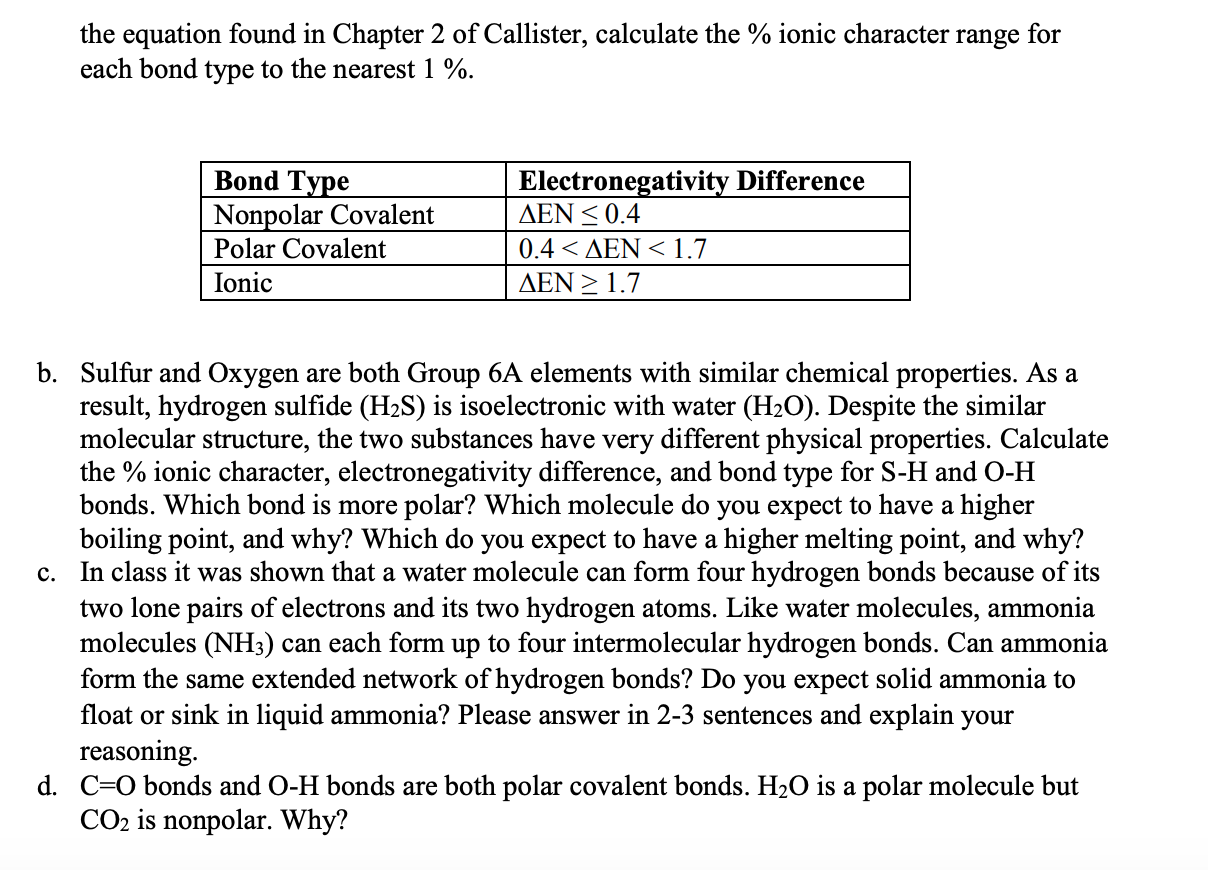
Solved The Larger The Electronegativity Difference En Chegg Com

8 3 Covalent Bonding Chemistry Libretexts
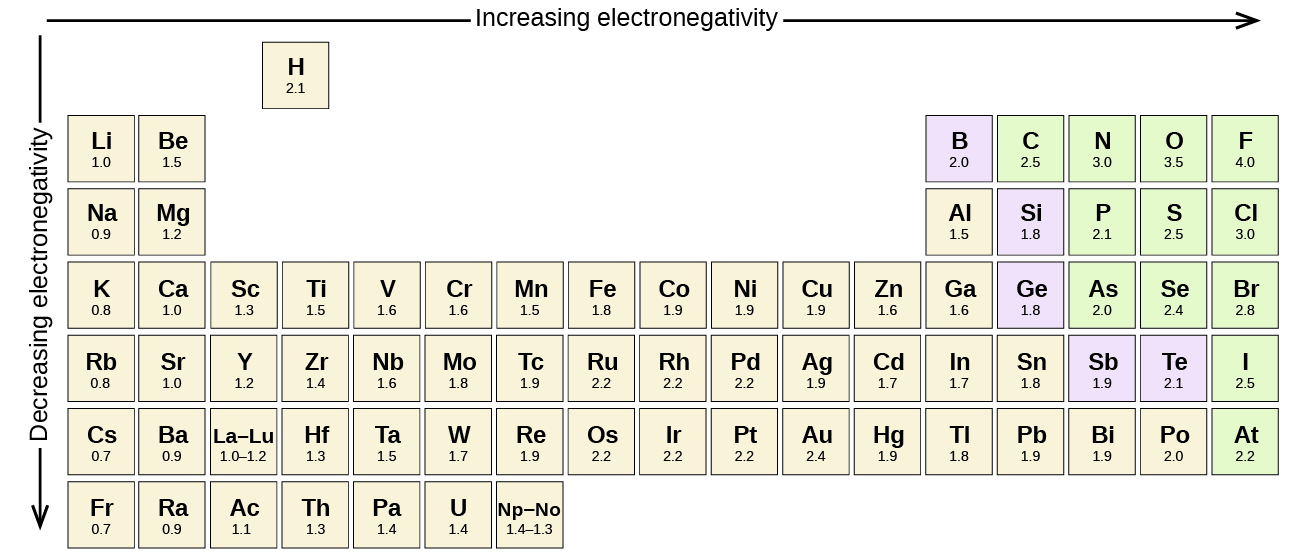
6 1 Electronegativity And Polarity Chemistry Libretexts

3 Ways To Calculate Electronegativity Wikihow

6 1 Electronegativity And Polarity Chemistry Libretexts

Bond Polarity Chemistry For Non Majors

Predicting Bond Type Electronegativity Video Khan Academy

Bond Polarity Chemistry For Non Majors
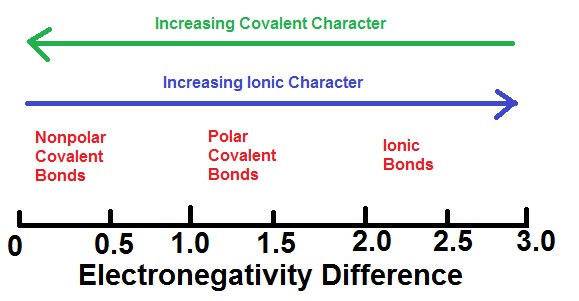
M8q1 Bonding And Electronegativity Chem 103 104 Resource Book

3 Ways To Calculate Electronegativity Wikihow
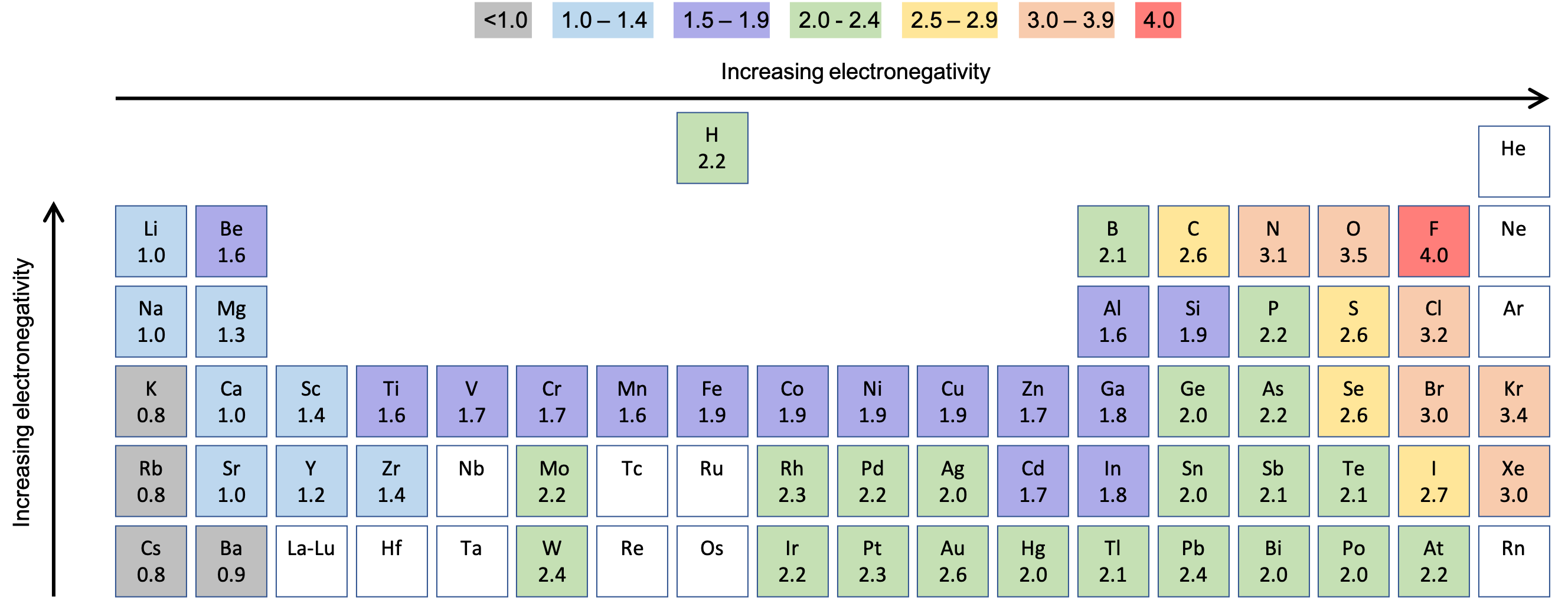
M8q1 Bonding And Electronegativity Chem 103 104 Resource Book

6 4 Polarity Of Molecules Introductory Chemistry
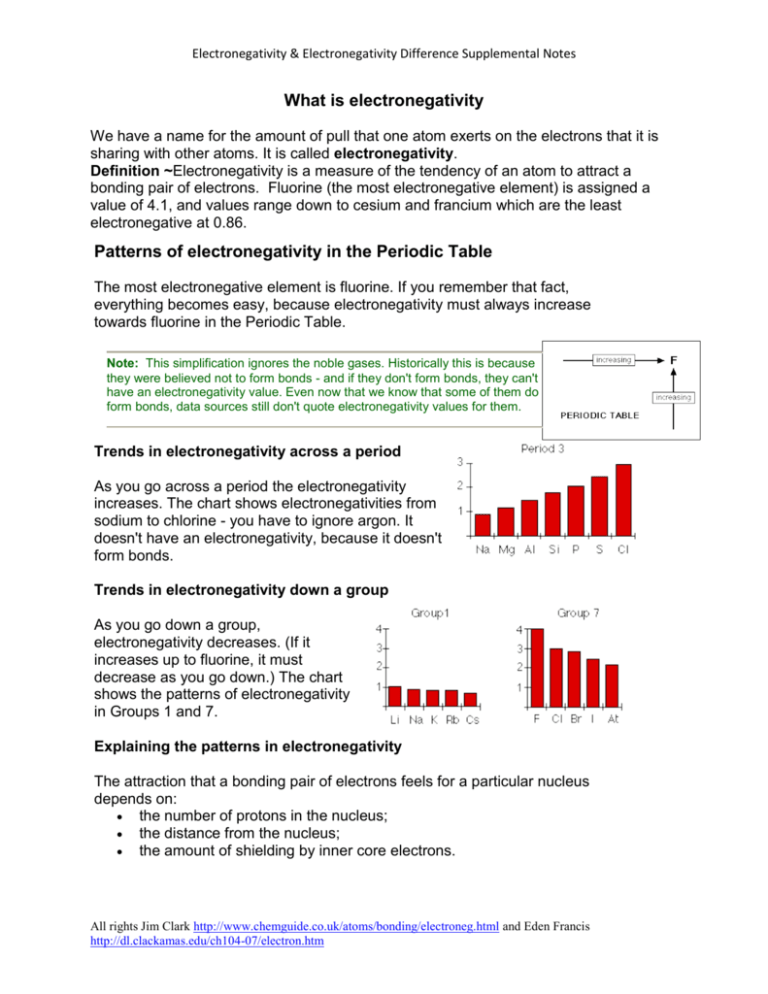
What Is Electronegativity Patterns Of Electronegativity In The Periodic

Question Video Determining Which System Has The Greatest Difference Of Electronegativity Values Nagwa

Lesson Explainer Electronegativity Nagwa

Solved Refer To Figure 10 2 To Find The Electronegativity Difference Between Each Pair Of Elements Then Refer To Table 10 2 To Classify The Bonds That Occur Between Them As Pure Covalent Polar Covalent

Electronegativity And Polarity Ppt Download
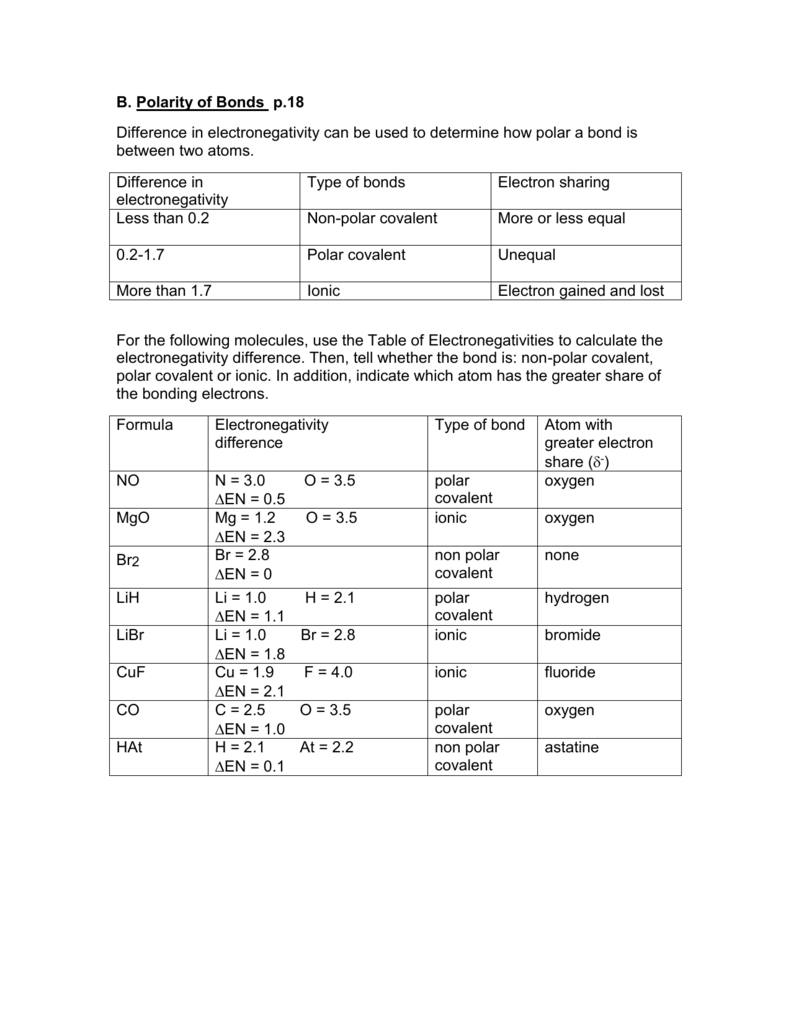
B Polarity Of Bonds P 18 Difference In Electronegativity Can Be Used
Comments
Post a Comment